The Cytophorics technology enables a variety of applications in the pharmaceutical industry, clinical diagnostics and basic research. Wherever a living cell sample is in interest of investigation, the patented sensor-equipped multi-well plate is able to capture high quality, relevant vital data in real time. The integrated fluidic system allows long-term studies for all kind of cell based assays. The main areas of interest are in personalized medicine in drug developing and in research to investigate basic cell mechanisms.
Personalized Medicine
Personalized medicine is the right treatment for the right person at the right time. Personalized medicine refers to the tailoring of medical treatment to the individual characteristics of each patient. The principle of adjusting treatment to specific patient characteristics has, of course, always been the goal of physicians. However, recent rapid advances in molecular biology are beginning to reveal a large number of possible new, genome-related, molecular markers for the presence of disease, susceptibility to disease, or differential response to treatment.
The Cytophorics technology allows direct studies of individual patient samples. Cell material that was obtained for example via a biopsy can be introduced into the patented sample carrier and supplied over the entire measurement period with nutrients and active agents. Individual statements regarding the toxicity of selected treatment strategies and their impact on the vitality of the patient samples can be shown.
Drug discovery screening
Pharmaceutical companies approach drug discovery in a variety of ways. An early part of the experimental process often involves screening a large number of compounds using defined biochemical assays in an ultrahigh-throughput format. However, the effect of a drug on an organism is complex and involves interactions at multiple levels that cannot be predicted using biochemical assays. Trying to understand this complexity has contributed to an increased use of high-content screening assays as more biologically relevant surrogates to predict the response of the organism. In addition, at some point in the drug discovery process, predicting cellular toxicity is important. Eukaryotic cell culture is accepted as the model system of choice to get a first approximation of toxicity. Furthermore, advances in assay chemistries and signal detection technology have allowed miniaturization of cell-based assays, making it more convenient to perform dose-response experiments even during primary screens.
Cytophorics provides an enabling technology for automated cell-based assay screenings for the pharmaceutical industry. Because of the high degree of automation also complex experiments with cells and tissues can be performed. Choosing a cell-based screening assay from among the available options requires understanding the endpoint measured, the correlation with cell viability, and the limitations of the assay chemistries. Through the real-time monitoring of vital parameters assay durations can be optimized and regenerative effects detected.
Establishing an In Vitro Model System
The species of origin and cell types used in cytotoxicity studies are often dictated by specific project goals or the drug target. Choosing a biologically representative cell line and appropriate assay conditions are important for providing relevant results. Regardless of the model system chosen, establishing a consistent and reproducible procedure for setting up assay plates is important. The number of cells per well and the equilibration period before the assay affects responsiveness to toxic compounds. Maintenance and liquid handling at each step of the process should be standardized and validated for consistency. Assay responsiveness to test compounds can be influenced by many subtle factors including culture medium surface-to-volume ratio, gas exchange, evaporation of liquids, and edge effects. These factors are especially important considerations when attempting to scale up assay throughput. Cytophorics offers a novel multiwell plate with up to 24 sample containers. With the patented sterile liquid handling system it is possible to automate the screening process.
No Endpoint needed 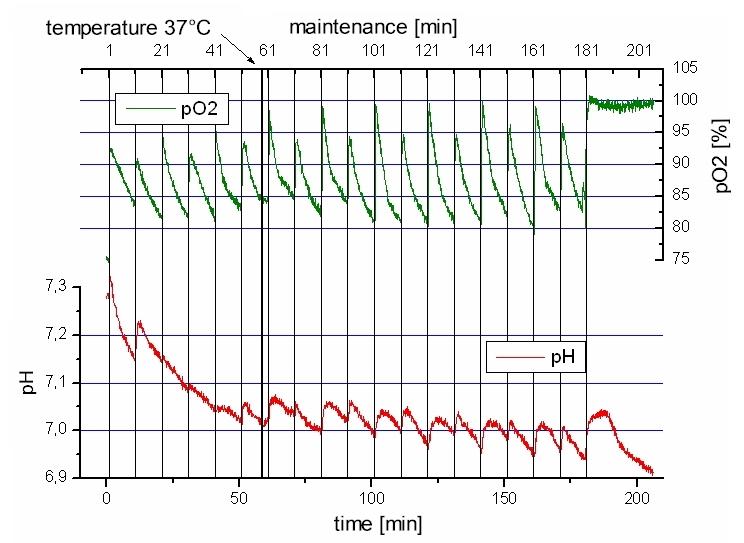
One of the first things to decide before choosing an assay format is exactly what information you want to measure at the end of a treatment period. Assays are available to measure a variety of different markers that indicate the number of dead cells (cytotoxicity assay), the number of live cells (viability assay), the total number of cells, or the mechanism of cell death (e.g., apoptosis).
A basic understanding of the changes that occur during different mechanisms of cell death will help in deciding which endpoint to choose for a cytotoxicity assay. Apoptotic cells in culture eventually undergo secondary exposure most of the population of cells are in a state of secondary necrosis. The Cytophorics technology enables a real time analysis. End points can be determined e.g. by assay development.
Picture: metabolism of one cell sample recorded for hours.
Further fields of Applications
- Toxicity testing
- Toxicokinetics
- Oncology: individual chemosensitivity tests
- 3D cell tests (spheroids)
- Regenerative medicine
- Food testing
- FCS Tests
- REACH-Tests
- Cyto-/Histopathology